Welcome to Dongguan Yinso Medical Packaging Co.,Ltd.
Welcome to Dongguan Yinso Medical Packaging Co.,Ltd.

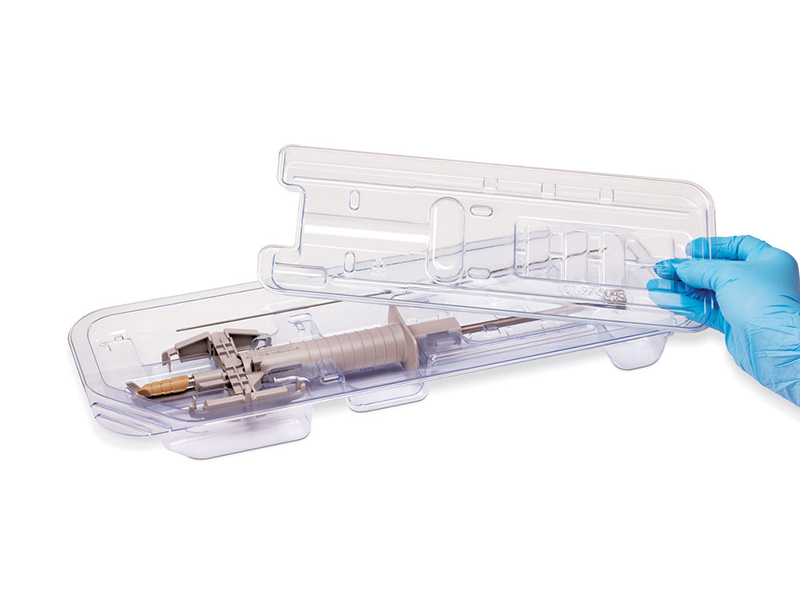
As a professional medical blister box manufacturer, we deeply understand the critical impact of customizing medical blister packaging on sterilization efficacy. Today, we will explore the differences between single-layer and dual-layer sterile barrier systems and share insights on optimizing blister box design to ensure sterilization compliance and product safety.
Single-Layer Barrier System: Simple Process, Low Validation Cost
Suitable for low-risk, short-term-use medical devices.
Ethylene Oxide (EO) sterilization demonstrates good penetration, requiring no extended aeration time.
Validated sterilization parameters can be directly reused, reducing additional validation costs.
Dual-Layer Barrier System: Enhanced Protection but Greater Sterilization Challenges
Suitable for high-risk, long-term implantable, or complex devices (e.g., cardiovascular stents, orthopedic implants).
EO sterilization penetration becomes more difficult, necessitating adjustments to process parameters:
Deeper vacuum cycles;
Longer sterilization and aeration times;
Increased nitrogen purging;
Potential increase in EO residue risk, requiring additional biocompatibility testing.
Packaging Size Compatibility
Avoid "bulging" risks: The breathable area of the secondary packaging (outer box) should be ≥ that of the primary packaging (inner box) to ensure pressure balance during vacuum cycles and prevent seal failure.
The breathable membrane design of the blister box must align with the sterilization method (e.g., high-breathability materials for EO sterilization).
Key Points for Sterilization Validation
Biological Indicator (BI) Placement: Place BIs both inside the primary packaging and outside the secondary packaging to ensure sterilization efficacy.
EO Residue Testing: Dual-layer packaging may increase residue risks, requiring strict monitoring and compliance with ISO 10993-7 standards.
Accelerated Aging Testing (ASTM F1980): Validate the seal integrity and sterile barrier performance of the packaging after long-term storage.
Material Selection Recommendations
Use high-breathability materials (e.g., Tyvek®) to ensure EO gas penetration.
Alternatively, consider puncture-resistant, moisture-proof medical-grade paper-plastic bags or aluminum composite films for additional protection.
Customized Packaging Design: Recommend single-layer or dual-layer barrier solutions based on device characteristics.
Sterilization Compatibility Testing: Assist in optimizing blister box structure to ensure EO/radiation sterilization efficacy.
Comprehensive Validation Support: Provide packaging integrity testing (ASTM F2096), breathability testing, and more to ensure compliance with ISO 11607 standards.
The design of the sterile barrier system for customizing medical blister packaging directly impacts sterilization efficacy and product safety. Single-layer packaging is suitable for most conventional devices, while dual-layer systems are better suited for high-risk, high-value products. However, additional attention must be paid to sterilization process adjustments and validation costs.
info@yinsopack.com
+86 15014837000(Wechat/WhatsApp/Skype)
NO.59 Meilin Road, Dalingshan Town, Dongguan City,Guangdong Province, China
Get a Free Prototype + Compliance Report Within 5 Days

Yinso Packing has been a leading figure in the medical packaging industry since 2007. We are committed to providing reliable, cost - effective, and innovative rigid thermoformed packaging solutions to top medical companies.
© 2025 Dongguan Yinso Medical Packaging Co.,Ltd All Rights Reserved.
AddressNO.59 Meilin Road, Dalingshan Town, Dongguan City,Guangdong Province, China
Emailinfo@yinsopack.com
Tel(Wechat/WhatsApp/Skype)+86 15014837000
About Us Medical Blister Packs Products Capabilities Blogs Contact Us